Published by: UNOY GmbH
AI-Assisted
Software MDR
Knowledge App [KAPP]
We created a KAPP for software development companies in the medical and life science industry to comply with Medical Devices Regulation. This tool identifies if software falls under the regulation and how to categorize it.

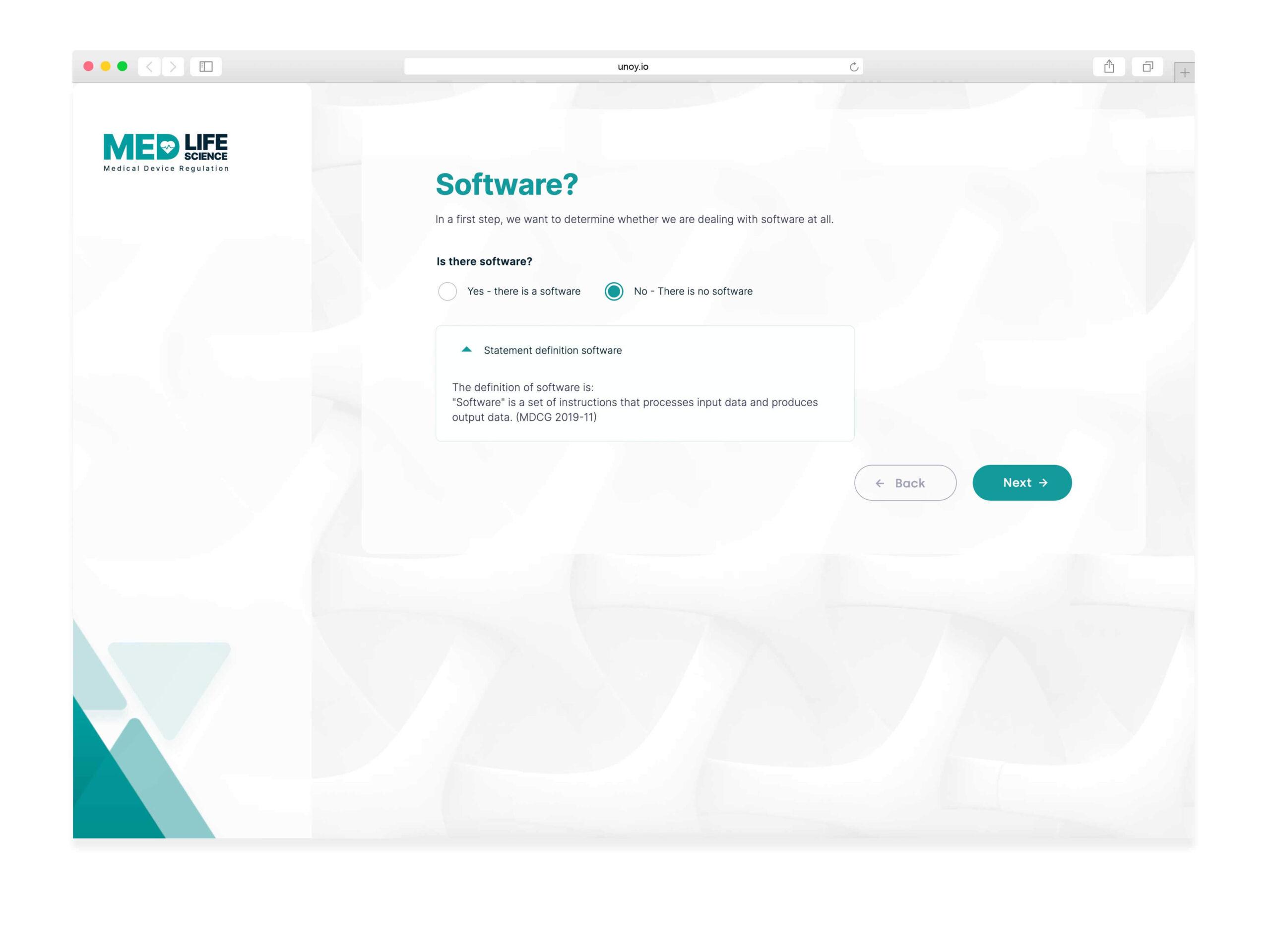

KAPP: Level 2
Author: UNOY Team
What the AI-Assisted MDR KAPP offers you:
- Minimizes risk in the Medical and Life Sciences industries
- Navigates the complex regulatory landscape
- Turns expert compliance and regulatory knowledge into interactive self-service tool
- Improves compliance by ensuring that users are up-to-date
- Reduces costs associated with non-compliance or regulatory mistakes
- Increases customer trust and confidence
Works perfect on:
Desktop
Tablet
Phone
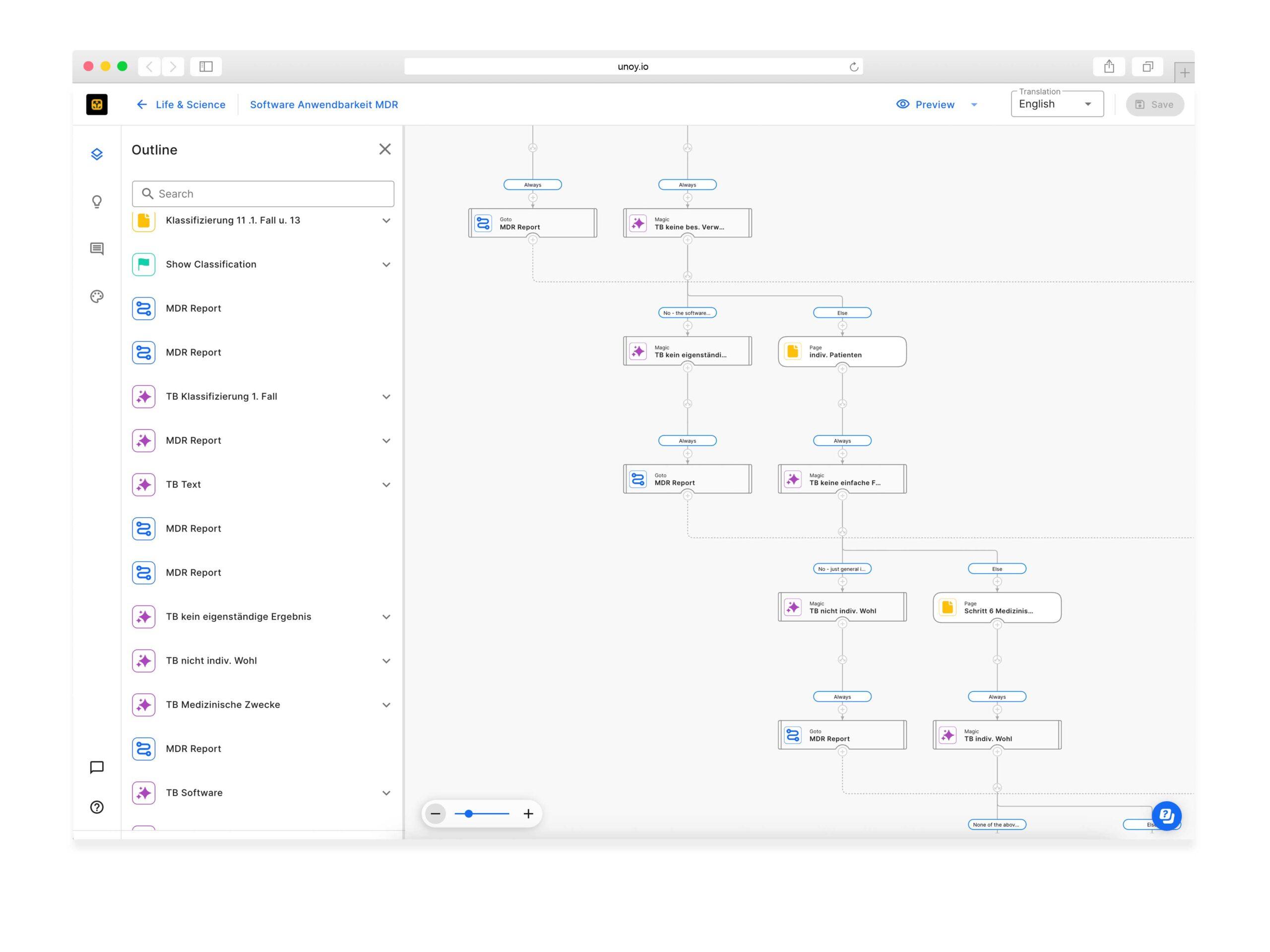
UNOY Designer vs. User Interface
Drag the yellow arrows to see the difference.

It’s time to go beyond manual knowledge work!
UNOY gives you the simplicity of No-Code but the power of a development team.
Get your daily work to another level with A.I. support!
Get your daily work to another level with A.I. support!
Get your daily work to another level with A.I. support!
Get your daily work to another level with A.I. support!
Get your daily work to another level with A.I. support!



